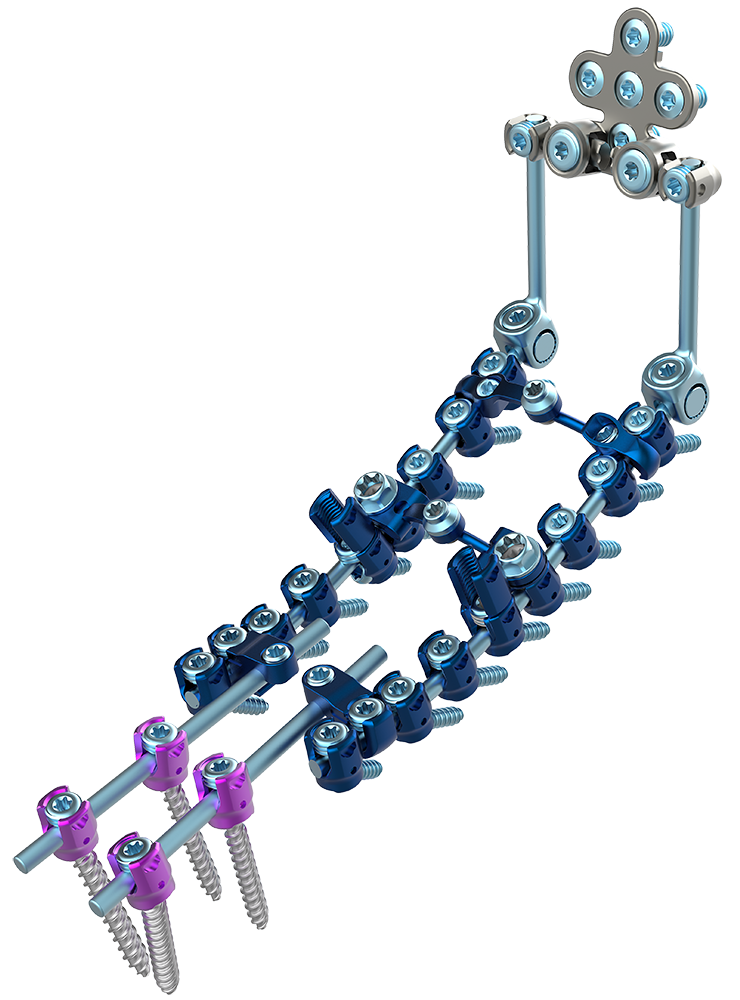
Astura Medical Announces Initial Cases and Full Commercial Release For BRIDALVEIL OCT Stabilization System
Astura Medical, a high growth, innovative spine technology company, announced today the completion of the initial surgeries and full commercial release for its BRIDALVEIL Occipital-Cervico-Thoracic (OCT) System. The first cases were successfully completed at multiple hospitals across the country, including at the University of Colorado Hospital by Dr. David Ou-Yang, Assistant Professor of Orthopedics.
“Our practice addresses a wide range of complex conditions, so I’m always looking for the most advanced technology to meet our surgical demands,” said Dr. Ou-Yang. “Bridalveil OCT has not only met those demands, but it has continued to exceed my expectations. The versatility of the instrumentation, coupled with the breadth of implants offered has enhanced our ability to treat even the most complex cases. It is the most comprehensive and one of the most user friendly posterior cervical systems I’ve used from any company, large or small.”
“We are extremely proud of the feedback and success we’ve seen with Bridalveil OCT since our initial release of the system,” said Joel Gambrell, President and CEO of Astura Medical. “It allows us to continue to expand upon our goal of providing market leading technology to our growing network of surgeon and distributor partners.”
Designed with the most complex and demanding cases in mind, BRIDALVEIL OCT provides a comprehensive offering of screw options (single-lead, dual-lead, high-top, and smooth shank) ranging from 3.5mm to 5.5mm in diameter that are compatible with either a 3.5mm or 4.0mm rod in titanium or cobalt chrome. The system additionally provides an extensive line of connectors, transition rods, and instrumentation options to allow surgeons the ability to seamlessly transition across multiple segments of the spine.























