Press Releases
-

-
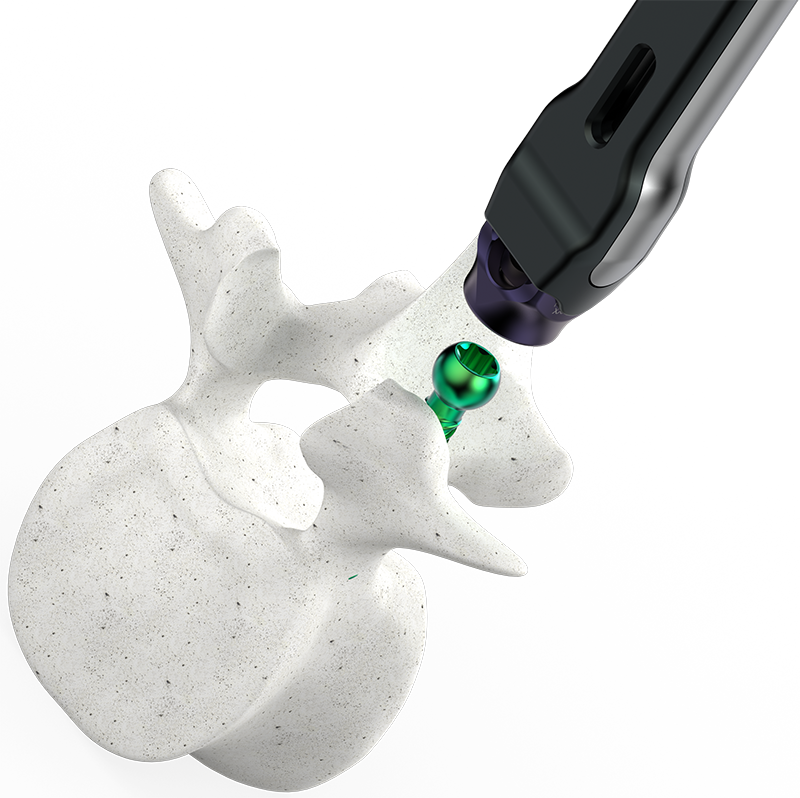
Astura Medical Receives FDA 510(k) Clearance For The Dominion Expandable Corpectomy System
Authored: March 11, 2024
IRVING, TX – February 16, 2024 –Astura Medical, a leader in innovative spinal surgery technologies, is proud to announce that it has received FDA 510(k) clearance for its Dominion Expandable Corpectomy System. Designed with patient outcomes in mind, the Dominion Expandable Corpectomy System features state-of-the-art, expandable columns with modular adjustable endplates that allow for a customizable anatomical fit, ensuring each procedure is as unique as the patient it serves. The system’s modular design and intuitive instrumentation facilitate a streamlined surgical …
-
-
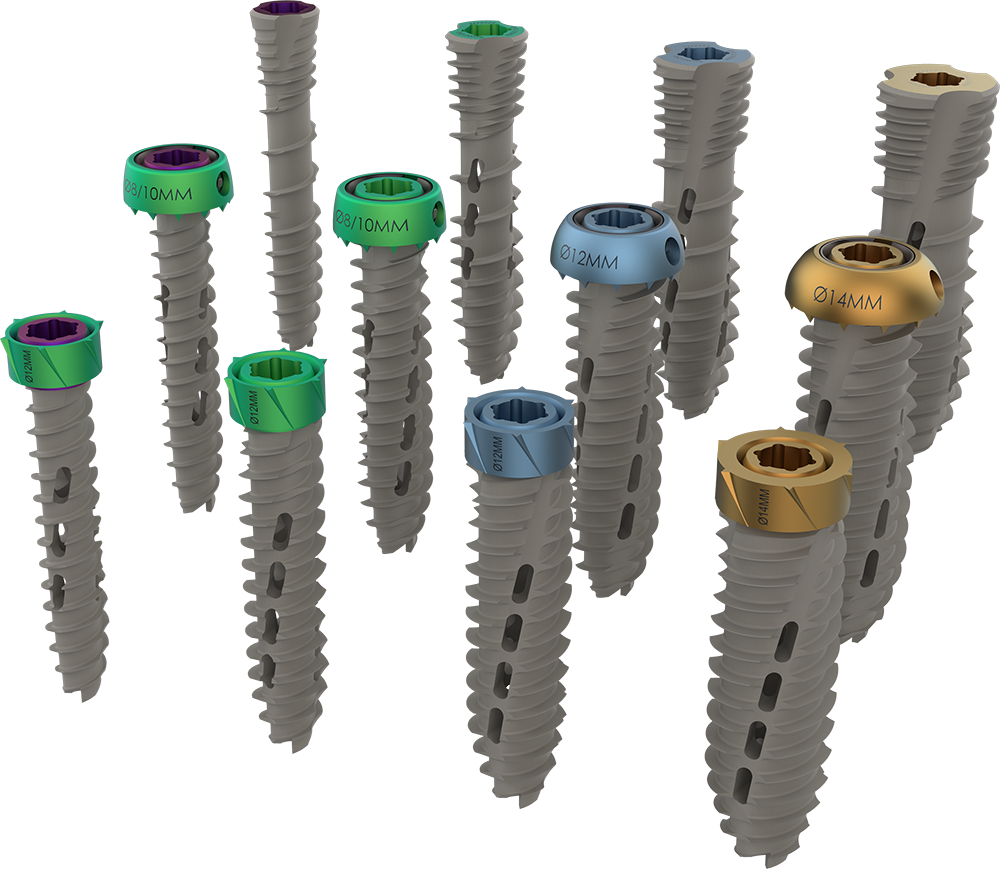
Astura Medical Announces FDA 510(k) Clearance For The Masada Modular Spinal Fixation System
Authored: November 23, 2023
Astura Medical, a dynamic spine technology company, is excited to announce that it has received FDA 510(k) clearance for its groundbreaking Masada Modular Spinal Fixation System. The Masada System is a versatile spinal fixation solution designed to empower surgeons and improve patient outcomes for complex spinal pathologies. It features a wide array of modular screw options with configurable tulips, offering translation, articulation, and rotation variability for enhanced surgical efficiency. Surgeons can easily adapt to individual patient anatomies using standard, extended-top, …
-
-
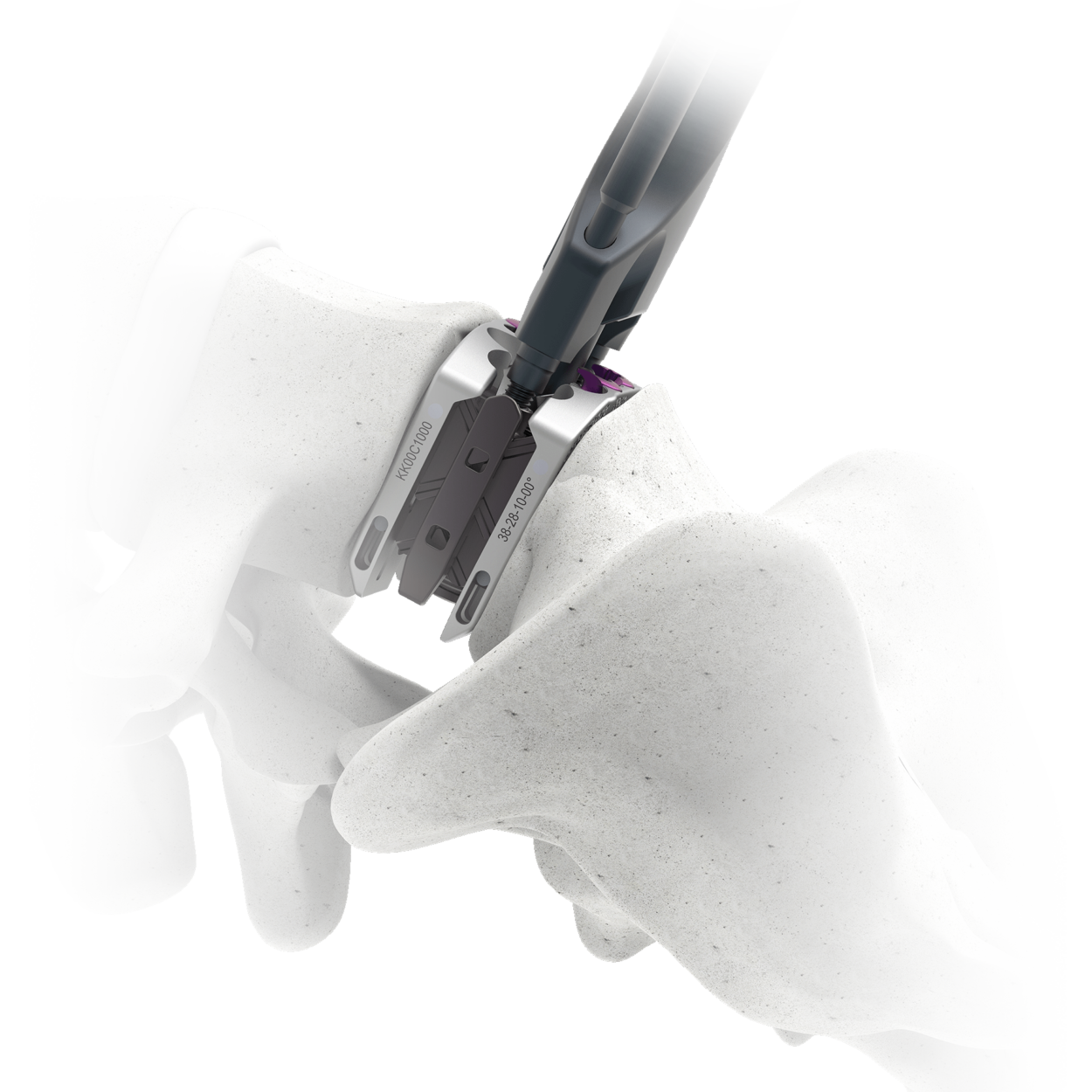
Astura Medical Receives FDA 510(k) Clearance For The Reunion Sacroiliac (SI) Joint Fusion System
Authored: November 14, 2023
Astura Medical, a leading spine technology company, is excited to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its innovative Reunion Sacroiliac (SI) Joint Fusion System. This advanced platform is designed for both minimally invasive lateral and posterior approaches for SI joint stabilization and fusion. It offers a wide range of instrumentation and modular screws for versatile surgical techniques. The Reunion system enables surgeons to perform SI joint fusion through two different …
-
-
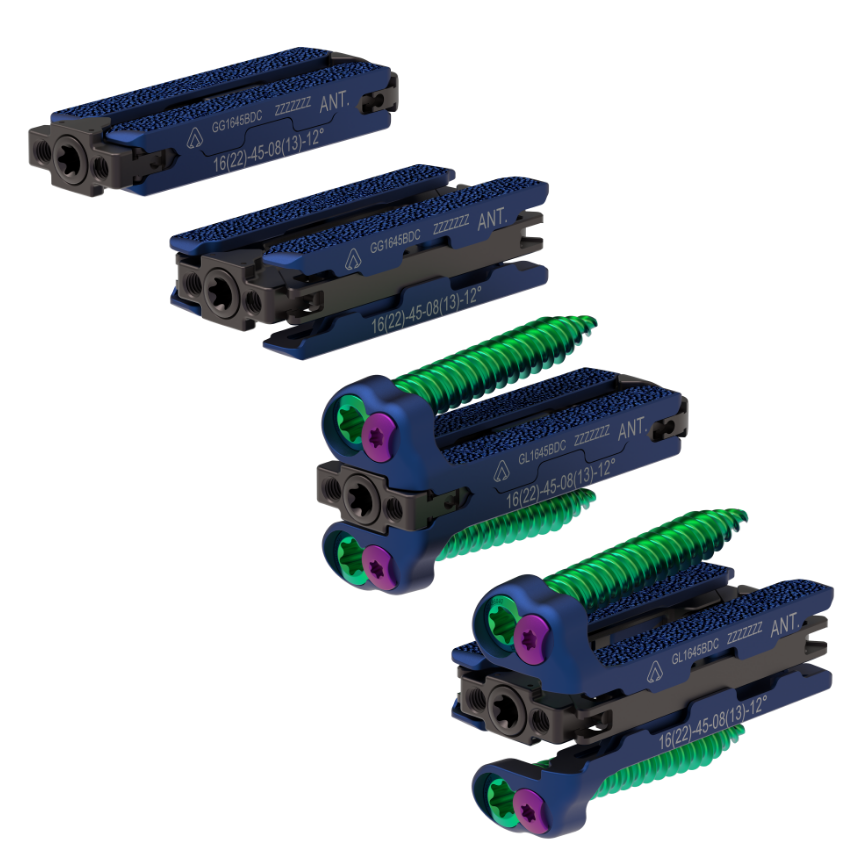
Astura Medical Announces Initial Case and Full Commercial Release For El Capitan X Expandable ALIF System
Authored: September 5, 2023
Astura Medical, a rapidly growing and innovative spinal device technology company, announced the full commercial release and first case with the company’s most recent expandable technology, El Capitan X Expandable Anterior Lumbar Interbody Fusion (ALIF) System. The first case was successfully completed by Jason Taub, M.D. from Dallas Neurosurgical & Spine at the Methodist Hospital for Surgery in Addison. Building upon the foundation of the success of the company’s other integrated plate/spacer technologies and expandable interbody platforms, El Capitan X …
-
-
Astura Medical Receives FDA 510(k) Clearance For The Sirion X Expandable Lateral Lumbar Interbody Fusion System
Authored: May 9, 2023
IRVING, TX – Astura Medical, a rapidly growing and innovative spine technology company, is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary Sirion X Expandable Lateral Lumbar Interbody Fusion (LLIF) System. Sirion X has been designed to deliver an all-encompassing range of expandable anatomic spacers and fixation solutions, including the option of non-plated or two-hole implants with standard or hyperlordotic cages. The spacers feature biplanar expansion starting at …
-

-
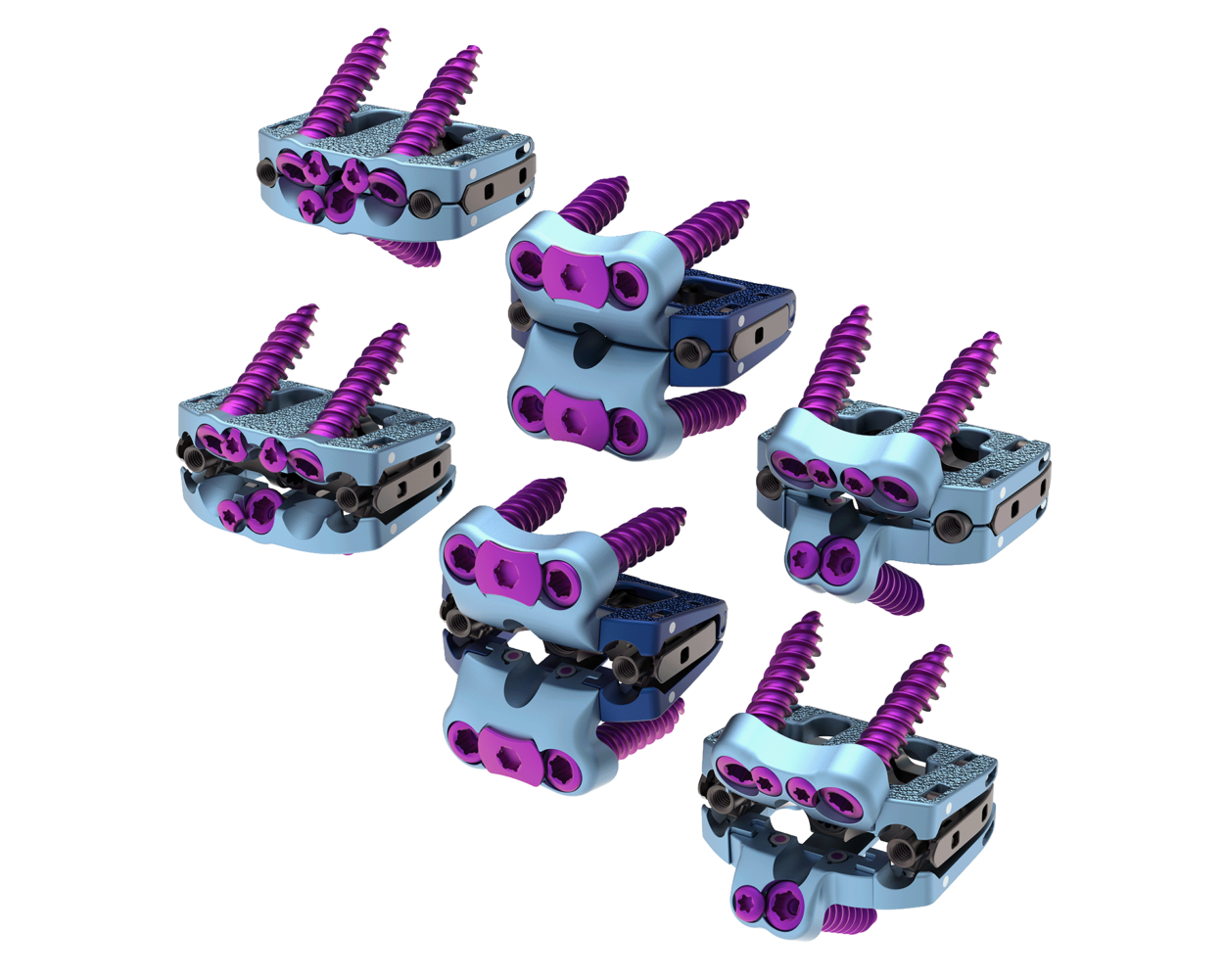
Astura Medical Receives FDA 510(k) Clearance For Olympic Deformity’s Sublaminar Bands Supplemental System
Authored: May 4, 2023
IRVING, TX – Astura Medical, a rapidly growing and innovative spine technology company, is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary Sublaminar Bands, which will serve as a supplementary set to the Olympic Deformity Posterior Spinal Deformity System. Building upon the technological advancements achieved with the Olympic Deformity System, Astura’s Sublaminar Bands Set provides surgeons with an alternative way to stabilize and correct spinal deformities by fastening …
-
-
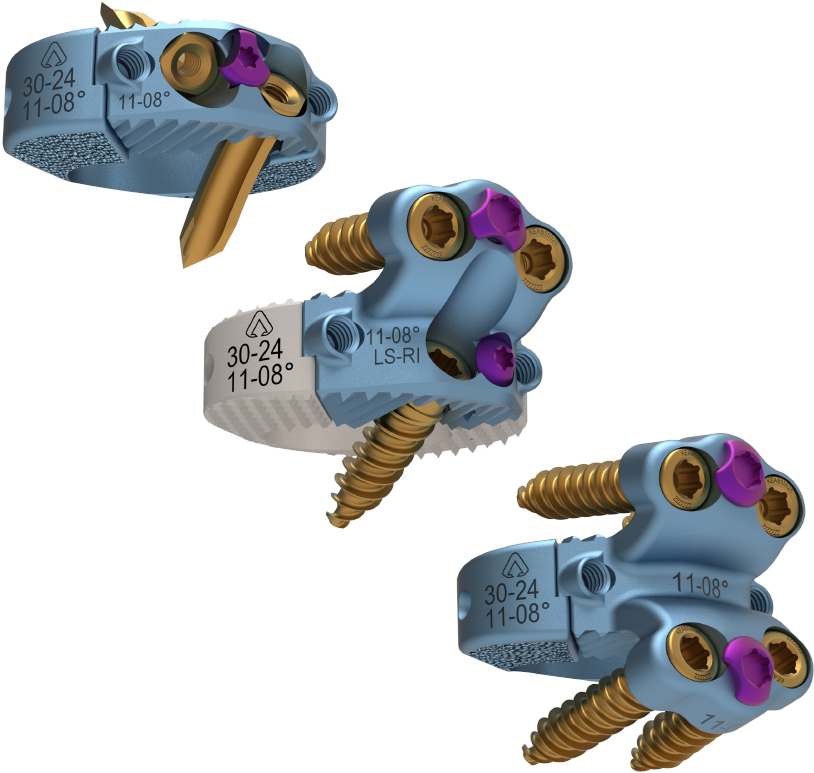
Astura Medical Receives FDA 510(k) Clearance For El Capitan X Expandable Anterior Lumbar Interbody System
Authored: January 20, 2023
IRVING, TX – January 20, 2023 – Astura Medical, a high-growth, innovative spine technology company, today has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan X Expandable Anterior Lumbar Interbody System. Constructed on the foundation of the success of the company’s integrated plate/spacer technologies and expandable interbody platforms, El Capitan X is a leap forward for what is now possible for anterior lumbar interbody fusion (ALIF) systems. The system …
-
-
Astura Medical Receives FDA 510(k) Clearance For El Capitan Oblique Anterior Lumbar Interbody Fusion System
Authored: April 4, 2022
Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan Oblique Anterior Lumbar Interbody Fusion (ALIF) System. Built from the foundation of success with the company’s other integrated plate and spacer technologies currently addressing anterior cervical, lateral lumbar, and traditional anterior lumbar approaches, El Capitan Oblique was specifically designed to deliver the widest array of construct options with the most efficient and …
-

-
Astura Medical Expands Sales Management Team
Authored: January 18, 2022
IRVING, TX – January 18, 2022 Astura Medical, a high-growth, innovative spine technology company, announced today the addition of Tom Frazer as Area Vice President of Sales, Midwest, and Brett Heim as Area Vice President of Sales, Northeast. “We’re thrilled to welcome Tom and Brett to the Astura Medical Team,” said Steve Haayen, Vice President of Commercial Operations. “The wealth of expertise that both gentlemen bring across multiple facets of the spine marketplace, combined with their leadership skills will be …
-

-
Astura Medical Receives FDA 510(k) Clearance For Dolomite Stand-Alone Anterior Cervical Stabilization System
Authored: November 13, 2020
Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Dolomite Stand-Alone Anterior Cervical Stabilization System. Expanding on the rapid adoption and recent success of the company’s other integrated plate and spacer technologies for anterior lumbar and lateral lumbar procedures, Dolomite was designed to deliver the widest array of construct options with the most efficient and streamlined supporting instrumentation in the anterior cervical surgery marketplace today. “Even …























