Astura Medical Receives FDA 510(k) Clearance For El Capitan Oblique Anterior Lumbar Interbody Fusion System
Astura Medical, a high-growth, innovative spine technology company, today announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan Oblique Anterior Lumbar Interbody Fusion (ALIF) System.
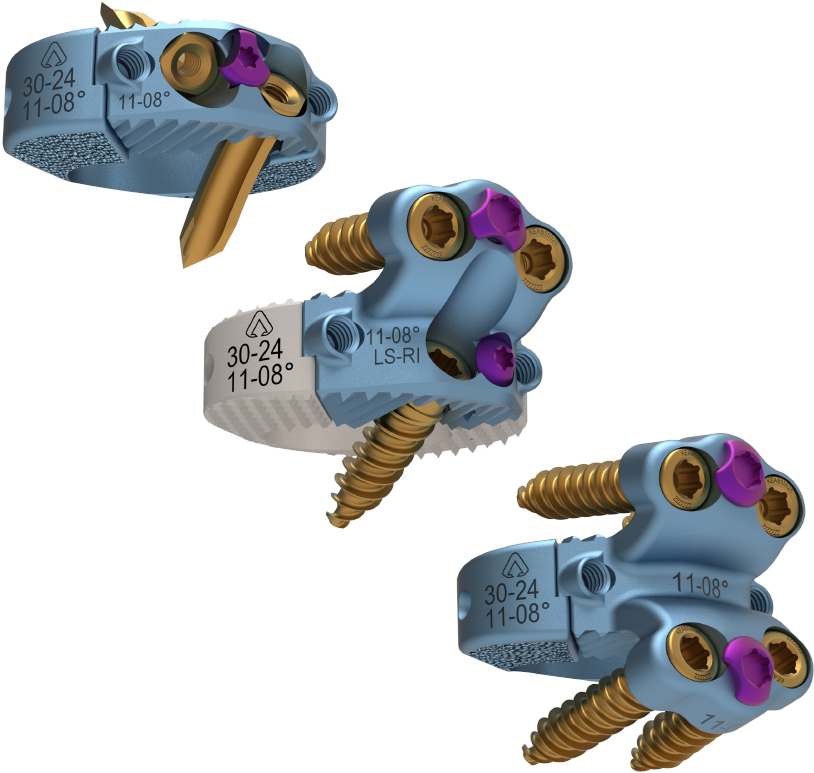
Built from the foundation of success with the company’s other integrated plate and spacer technologies currently addressing anterior cervical, lateral lumbar, and traditional anterior lumbar approaches, El Capitan Oblique was specifically designed to deliver the widest array of construct options with the most efficient and streamlined supporting instrumentation in the oblique anterior lumbar surgery marketplace today.
“With the rising demand for technology to provide anterior column reconstruction beyond traditional anterior and lateral lumbar approaches, we set out to provide a system that would establish a higher benchmark for intraoperative versatility, flexibility, and efficiency in oblique anterior lumbar surgery,” said Thomas Purcell, Co-founder and Vice President. “Thanks to the hard work and ingenuity of our engineering team and surgeon design partners, we were able to achieve our goal by delivering another best-in-class technology to our portfolio of solutions.”
In an effort to tailor to the specific needs of each patient, El Capitan Oblique is the first system to provide multiple, customizable plating and fixation options in oblique anterior lumbar procedures. The plating options from El Capitan Oblique allow for either a Zero-Profile, Half Plate, or Full Plate that can be implanted in combination with the interbody from either a left or right sided approach. By providing either Anchoring Nails or Screws, which can be delivered via multiple techniques including an All-in-One option, El Capitan Oblique can accommodate the wide variance of anatomical challenges presented in oblique anterior lumbar surgery.
El Capitan Oblique is the company’s fourth technology platform providing a combined interbody and fixation option to receive 510k approval. For more information on the El Capitan Oblique ALIF System, click here.
Astura Medical was formed in 2014 with the objective of creating a disciplined, multi-phased approach to developing, manufacturing, and distributing medical devices. With surgeon input and feedback at every stage of development, Astura has created an extensive line of devices of the highest quality and sleekest design.
The two essential pillars that contribute to Astura Medical’s success are high quality products and robust distribution channels. These pillars, combined with passion and innovation, are what drive the Astura team to achieve great success with developing devices and entering them into the marketplace.























