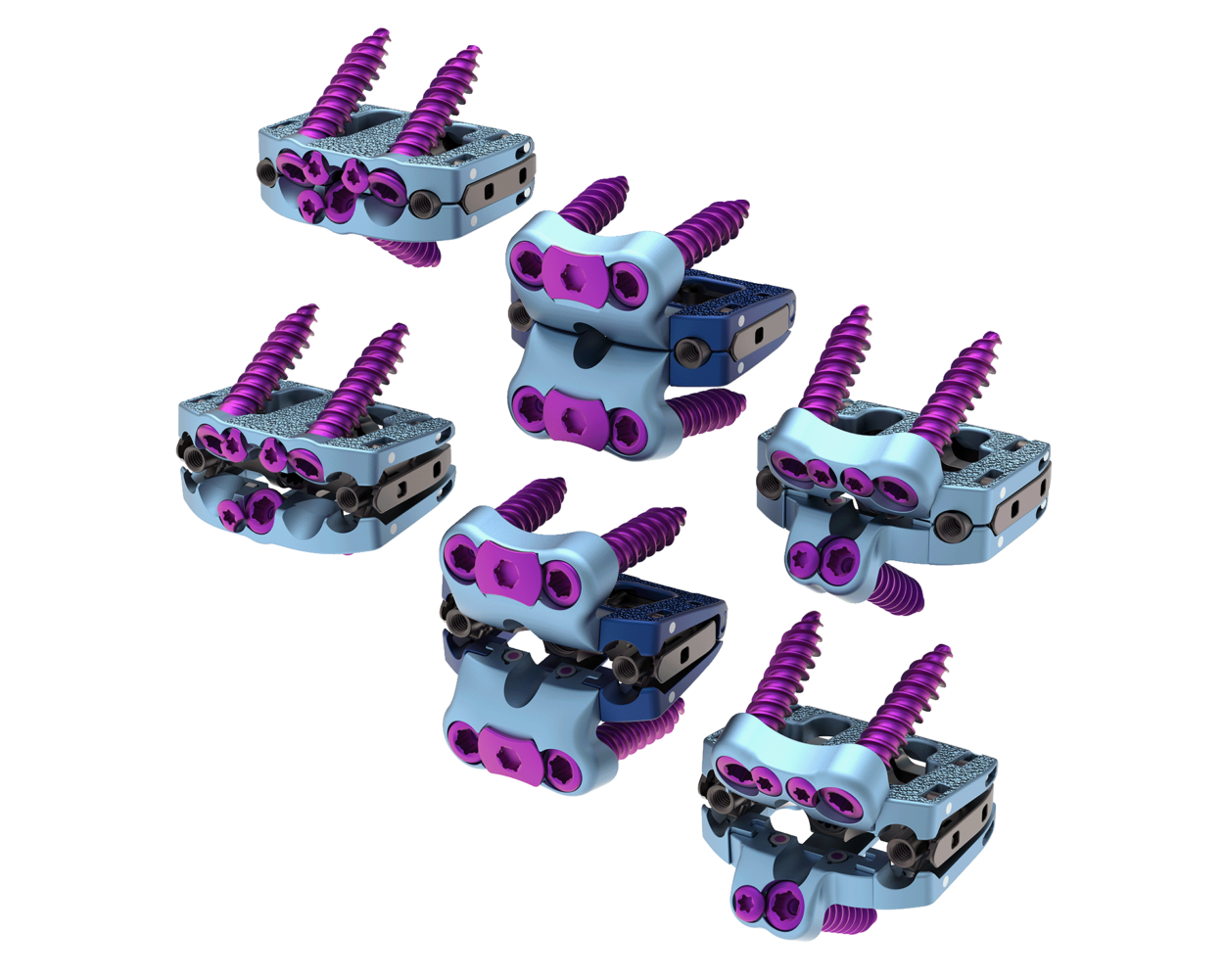
Astura Medical Receives FDA 510(k) Clearance For El Capitan X Expandable Anterior Lumbar Interbody System
IRVING, TX – January 20, 2023 – Astura Medical, a high-growth, innovative spine technology company, today has announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its El Capitan X Expandable Anterior Lumbar Interbody System.
Constructed on the foundation of the success of the company’s integrated plate/spacer technologies and expandable interbody platforms, El Capitan X is a leap forward for what is now possible for anterior lumbar interbody fusion (ALIF) systems. The system provides a complete range of expandable anatomic spacers and fixation options with infinitely adjustable expansions in height and lordosis up to 30° for a unique, patient-specific solution.
El Capitan X’s intuitive design delivers a comprehensive range of infinitely adjustable construct options and expansive instrumentation that provide the versatility to address even the most complex anatomical challenges in a streamlined, efficient procedural sequence.
“The approval of El Capitan X represents a new benchmark for what’s possible in the anterior lumbar fusion marketplace,” said Thomas Purcell, Co-founder and Vice President. “By becoming the first and only system to combine integrated plate and spacer options, along with customizable expandable interbody capabilities, the system delivers on our goal of providing more intraoperative flexibility and versatility than any other ALIF system on the market. El Capitan X is redefining what it means to deliver a truly patient-specific solution.”
El Capitan X is Astura Medical’s 1st expandable technology platform with multiple fixation configurations to receive 510k approval. The company is scheduled to release El Capitan Oblique ALIF later this spring as well as introduce several new, expandable technologies in the coming months.
For more information, please visit El Capitan X or LinkedIn.
About Astura Medical
Astura Medical was formed in 2014 with the objective of creating a disciplined, multi-phased approach to developing, manufacturing, and distributing medical devices. With surgeon input and feedback at every stage of development, Astura has created an extensive line of devices of the highest quality and sleekest design.
The two essential pillars that contribute to Astura Medical’s success are high-quality products and robust distribution channels. These pillars, combined with passion and innovation, are what drive the Astura team to achieve great success with developing devices and entering them into the marketplace.























