Astura Medical Receives FDA 510(k) Clearance For The Sirion X Expandable Lateral Lumbar Interbody Fusion System
IRVING, TX – Astura Medical, a rapidly growing and innovative spine technology company, is proud to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its revolutionary Sirion X Expandable Lateral Lumbar Interbody Fusion (LLIF) System.
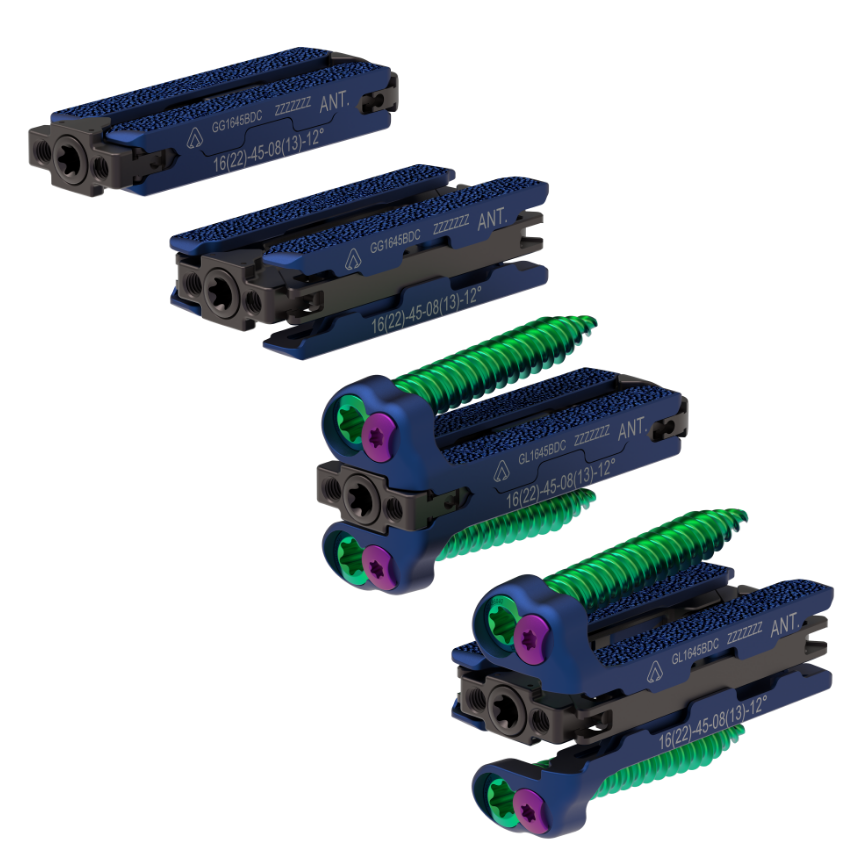
Sirion X has been designed to deliver an all-encompassing range of expandable anatomic spacers and fixation solutions, including the option of non-plated or two-hole implants with standard or hyperlordotic cages. The spacers feature biplanar expansion starting at 16mm anterior/posterior (A/P) to minimize retraction forces within the psoas and expanding up to 22mm A/P, as well as increasing in height by up to 5mm and up to 25º of lordosis. This allows for a controlled disc height restoration and an optimized fit while reducing the risk of iatrogenic endplate disruption.
The implant features acid-etched endplates and an integrated bone graft delivery system that delivers higher volumes of graft material and better endplate contact. The comprehensive selection of implants and related instruments has been designed to streamline the surgical process, offering surgeons the ability to confront even the most challenging circumstances through multiple techniques.
“We are thrilled to announce the approval of Sirion X, which represents a significant milestone for our surgeon designers and engineering team. Our dedication to creating patient-specific solutions has culminated in a market-leading system in terms of intraoperative flexibility and versatility,” said Joel Gambrell, Co-founder, and CEO.
Sirion X is the second expandable technology to be granted 510(k) approval by the FDA in 2023, following El Capitan X Expandable ALIF in January.
For more information, please visit Sirion X or LinkedIn.
About Astura Medical
Astura Medical was formed in 2014 with the objective of creating a disciplined, multi-phased approach to developing, manufacturing, and distributing medical devices. With surgeon input and feedback at every stage of development, Astura has created an extensive line of devices of the highest quality and sleekest design.
The two essential pillars that contribute to Astura Medical’s success are high-quality products and robust distribution channels. These pillars, combined with passion and innovation, are what drive the Astura team to achieve great success with developing devices and entering them into the marketplace.
























