Astura Medical Receives FDA 510(k) Clearance For The Reunion Sacroiliac (SI) Joint Fusion System
Astura Medical, a leading spine technology company, is excited to announce that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its innovative Reunion Sacroiliac (SI) Joint Fusion System.
This advanced platform is designed for both minimally invasive lateral and posterior approaches for SI joint stabilization and fusion. It offers a wide range of instrumentation and modular screws for versatile surgical techniques.
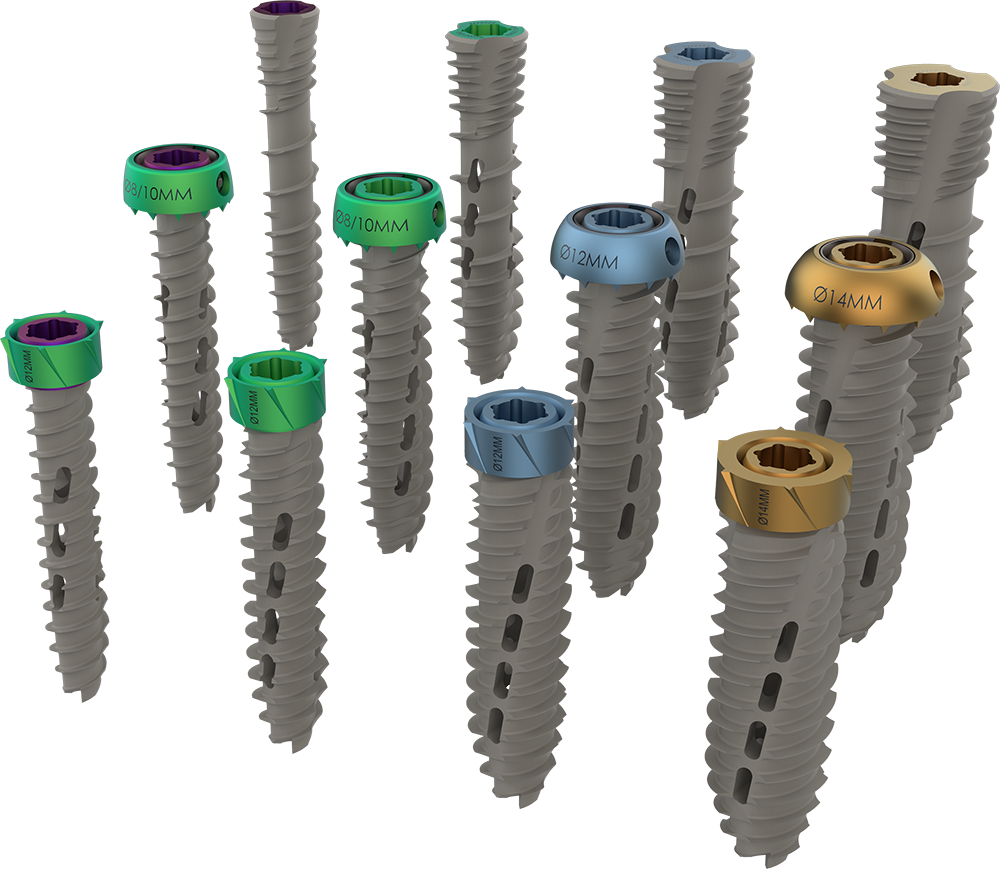
The Reunion system enables surgeons to perform SI joint fusion through two different approaches and offers a range of modular screws with diameters of 8, 10, 12, or 14mm and lengths from 35-80mm. It also provides two screw options, a modular head with a dual lead thread or a compression style head with a compression thread.
The system includes modular screw heads with an integrated automatic locking anti-rotation feature for secure attachment to the implant screws. The screw heads come in either a Flush Mount or Variable Surface Mount screw head, depending on the desired proximal anchoring technique.
Joel Gambrell, Co-founder, and CEO, said, “Reunion is the result of years of research and a commitment to improving the quality of life for SI joint disorder patients. We’re proud to offer a solution that enhances surgical precision and patient outcomes.”
In addition to the FDA clearance for the Reunion System, Astura Medical has several new technologies awaiting approval, including an expandable corpectomy cage and expandable articular TLIF. These innovations are expected to receive clearance in the coming months, positioning Astura Medical as a leading player in the spine industry.
For more information, visit www.asturamedical.com or find us on LinkedIn.
About Astura Medical
Astura Medical was formed in 2014 with the objective of creating a disciplined, multi-phased approach to developing, manufacturing, and distributing medical devices. With surgeon input and feedback at every stage of development, Astura has created an extensive line of devices of the highest quality and sleekest design.
The two essential pillars that contribute to Astura Medical’s success are high-quality products and robust distribution channels. These pillars, combined with passion and innovation, are what drive the Astura team to achieve great success with developing devices and entering them into the marketplace.
























